邮箱地址
前沿资讯
发布时间:2024-02-26 09:42:31 作者:青岛海大海洋寡糖科技有限公司 来源:本站
文章题目:Developing Next-Generation Protein-Based Vaccines Using High-Affinity Glycan Ligand-Decorated Glyconanoparticles
发表期刊 : Advanced Science
影响因子 : 17.521(2021)
通讯单位 : 山东大学国家糖工程技术研究中心
疫苗作为人类历史上最重要的公共卫生成就之一,自诞生以来大大降低了包括白喉、麻疹、腮腺炎、百日咳、脊髓灰质炎、风疹、破伤风和流感嗜血杆菌感染等多种疾病对人类健康的威胁。然而,由于大量新抗原的免疫原性较差,疫苗在治疗肿瘤以及新出现的传染性疾病方面仍然存在不足,为了改善疫苗治疗效果,迫切需要开发更有效的免疫治疗策略。在以往的研究中,免疫细胞对纳米颗粒疫苗的摄取属于被动方式,而通过主动方式将抗原高效递送至抗原递呈细胞则是一种增强免疫的重要方法。巨噬细胞是一类抗原递呈细胞,在免疫应答过程中发挥着重要的作用。在这篇文章中,作者基于乙氧基衍生化的缩醛化葡聚糖以及Siglec-1高亲和力糖基配体,开发了一种靶向巨噬细胞的新纳米疫苗体系,并将该纳米疫苗体系应用于肿瘤、新型冠状病毒疫苗的开发。
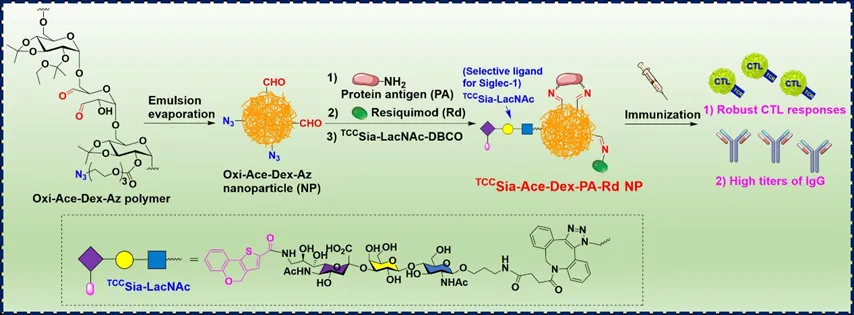
首先,可用于负载蛋白抗原、免疫佐剂和Siglec-1高亲和力配体的Oxi-Ace-Dex-Az NPs,是由Dextran(Dex)经过叠氮官能团化修饰、高碘酸氧化和乙氧基修饰获得缩醛化葡聚糖(Oxi-Ace-Dex-Az),通过双乳液法制备的(Figure 1a)。Siglec-1高亲和力配体TCCSia-LacNAcPro-DBCO则是以GlcNAcProN3为原料经过两步酶法糖基化修饰以及随后的TCC修饰和DBCO官能团化制备的(Figure 1b)。


Figure 1. a)Synthesis of Oxi-Ace-Dex-Az nanoparticles (NPs). b) Synthesis of TCCSia-LacNAc-DBCO.
获得Oxi-Ace-Dex-Az NPs和TCCSia-LacNAcPro-DBCO之后,作者通过醛基与氨基形成席夫碱的反应,将模型抗原和免疫激动剂R848通过共价键的方式连接到纳米颗粒表面,随后通过无Cu离子参与的环加成反应(SPAAC)在纳米疫苗表面继续安装Siglec-1的高亲和力配体TCCSia-LacNAc(Figure 2),从而获得能够有效靶向巨噬细胞的新纳米疫苗体系TCCSia-Ace-Dex-OVA-Rd。
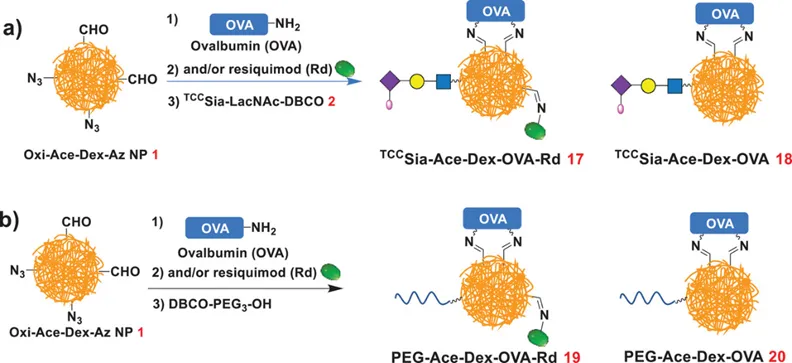
Scheme 1. Synthesis of TCCSia-Ace-Dex-OVA-Rd and TCCSia-Ace-Dex-OVA. b) Synthesis of PEG-Ace-Dex-OVA-Rd and PEG-Ace-Dex-OVA.
接下来,作者继续在细胞和动物水平上评价了TCCSia-Ace-Dex-OVA-Rd对巨噬细胞的靶向和细胞免疫激活作用。结果显示,连接有TCCSia-LacNAc配体的纳米疫苗能够更加有效的被BMMs摄取和促进抗原的呈递(Figure 2,3a and 3b)。除此之外,TCCSia-Ace-Dex-OVA-Rd纳米疫苗也表现出更加有效的体内特异性CTL杀伤作用(Figure 3 c-f),这说明该纳米疫苗诱导强大的细胞免疫
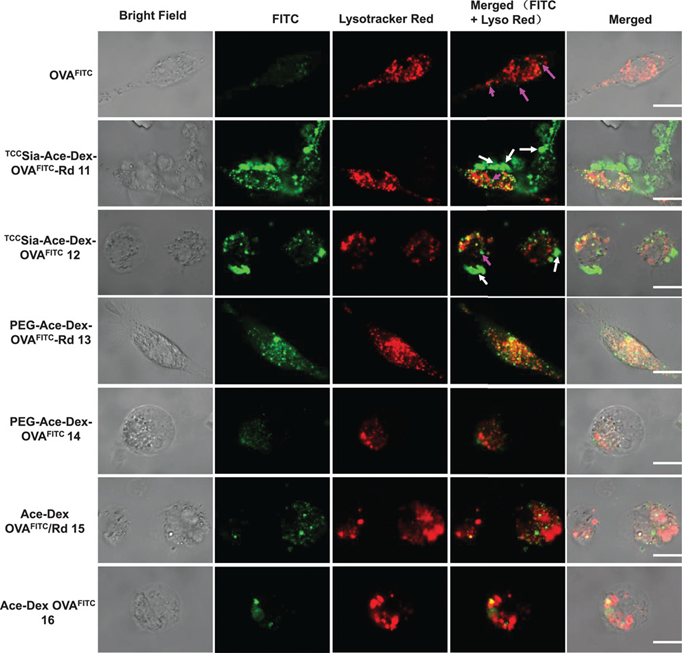
Figure 2.Colocalization of NPs within CD169+ bone marrow-derived macrophages (BMMs) upon incubation with the nanoparticles (NPs; containing the same amount of OVAFITC, 10 µg) for 6 h.
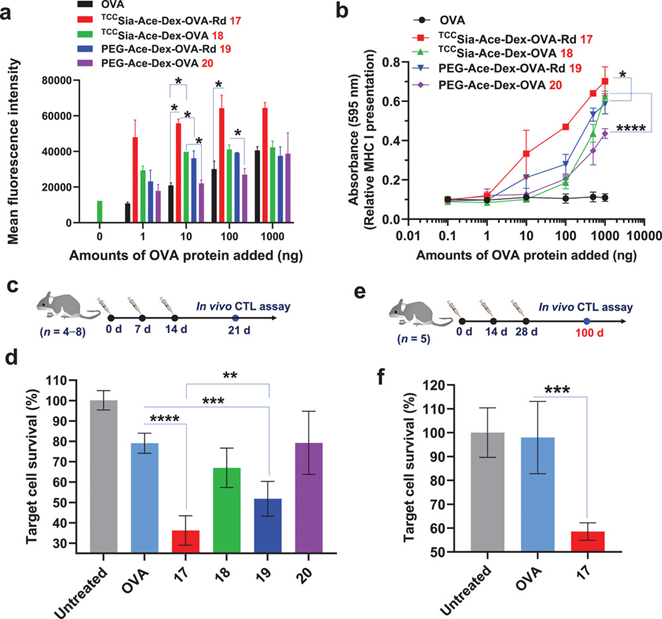
Figure 3. a) Detection of OVA257−264 presented by MHC-I of the bone marrow-derived macrophages (BMMs; CD169+). b) MHC-I antigen presentation by the BMMs. c,d) In vivo CTL activities. e,f) Persistence of in vivo CTL activities.
已经证明了TCCSia-Ace-Dex-OVA-Rd能够诱导强大的细胞免疫反应,作者继续检测TCCSia-Ace-Dex-OVA-Rd对体液免疫的激活作用。通过ELISA测定纳米疫苗的抗体滴度,结果显示TCCSia-Ace-Dex-OVA-Rd诱导了强大且持久的体液免疫(Figure 4)。
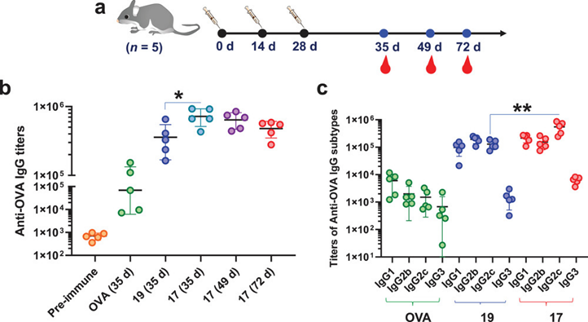
Figure 4. a,b) Anti-OVA IgG antibody titers in mice immunized with free OVA, TCCSia-Ace-Dex-OVA-Rd (17), and PEG-Ace-Dex-OVA-Rd (19), respectively. c) IgG subtype titers in mice immunized with free OVA, 19 or 17.
由于TCCSia-Ace-Dex-OVA-Rd出色的CTL和IgG反应,作者评估了其在EG7-OVA肿瘤模型中对肿瘤的抑制作用。总共接种三针疫苗后,TCCSia-Ace-Dex-OVA-Rd有效抑制了肿瘤的增长(Figure 5b-d),并且没有造成小鼠体重的下降(Figure 5e)。

Figure 5. EG7-OVA tumor growth. b) Growth curves of tumors in mice were collected. c) Photographs of the dissected tumors were taken, and d) the dissected tumors were weighed. e) The average body weight of tumor-bearing mice was monitored over time.
截至2022年10月6日,全球有616951418例COVID-19确诊病例,653281例死亡。为应对日益严峻的新冠疫情,以及进一步验证靶向纳米疫苗平台能够广泛应用于多种蛋白疫苗的开发,作者继续分别开发了携带有SARS-CoV-2重组RBD蛋白的TCCSia-Ace-Dex-RBD-Rd纳米疫苗。对纳米疫苗诱导产生的抗原特异性IgG抗体滴度检测发现,两种纳米疫苗在小鼠和兔子体内激发了更加高水平且长效的IgG抗体(Figure 6c-g and Figure 7b,c),并且有效抑制了真病毒对宿主细胞的感染(Figure 7d-f)。
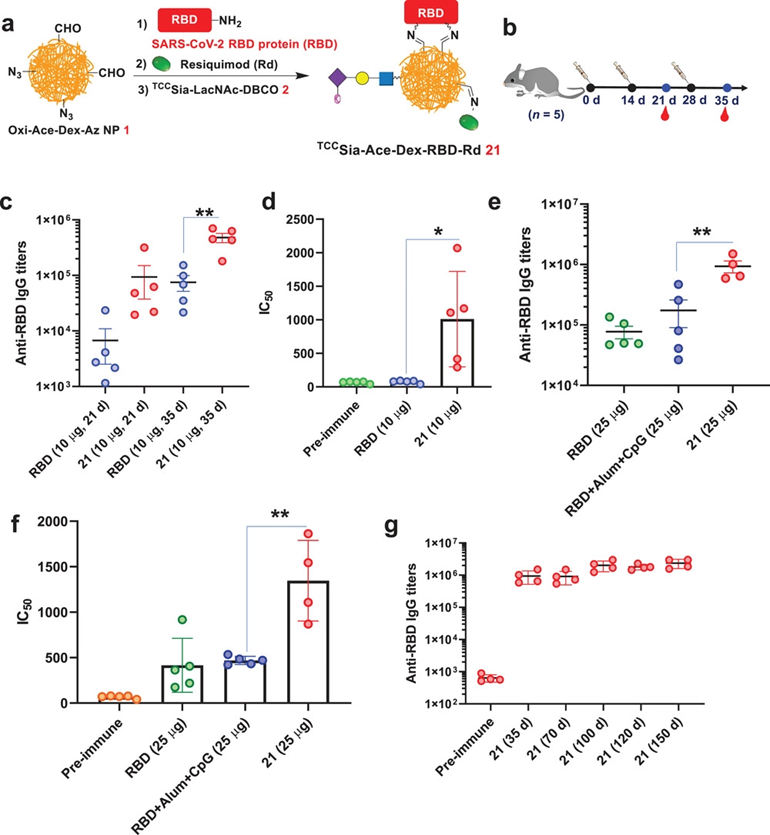
Figure 6. a) Synthesis of TCCSia-Ace-Dex-RBD-Rd (21). b) Immunization of mice with free receptor-binding domain (RBD) (10 µg) and 21 (with 10 µg RBD in the nanoparticles, NPs), respectively. c) Titers of anti-RBD IgG from mice immunized with free RBD and 21, respectively. d) IC50 values of RBD binding to hACE2 by antibodies elicited by free RBD and 21 showed 21-induced antibodies significantly blocked the SARS-CoV-2 RBD binding to hACE2. e–g) Immunization of mice with free RBD (25 µg), 21 (with 25 µg RBD in the NPs), or RBD+Alum+CpG (with 25 µg RBD).

Figure 7. a) Immunization of rabbits with free receptor-binding domain (RBD; 25 µg) and21 (with 25 µg RBD), respectively. b) Titers of anti-RBD IgG from rabbits immunized with free RBD and 21, respectively. c) IC50 values of RBD binding to hACE2 by IgG elicited by free RBD and 21 from rabbits showed 21-induced IgG effectively blocked SARS-CoV-2 RBD binding to hACE2. infection of Vero E6 cells. b,c) Each symbol represents one rabbit. d–f) Authentic SARS-CoV-2 neutralization test showed that 21 postimmune rabbit sera effectively neutralized authentic SARS-CoV-2 (WIV04 strain) virus infection of Vero E6 cells.
原文链接:
https://doi.org/10.1002/advs.202204598
作者:汪浩
审核:李全才,吕友晶
编辑:邵萌
如有侵权,请联系删除